
eHealth Africa provided operational and logistical support to the Ministry of Health and Sanitation (MoHS), and the U.S. Centers for Disease Control and Prevention (CDC) for a pilot two day training session for MoHS swabbers and surveillance officers in Western Area and Kenema, Sierra Leone. The training focused on the implementation of Orasure’s OraQuick Ebola Rapid Screening Test (RST) in routine dead body swabbing. This pilot is being implemented to inform an upcoming national rollout of the OraQuick RST, scheduled for February or March this year.
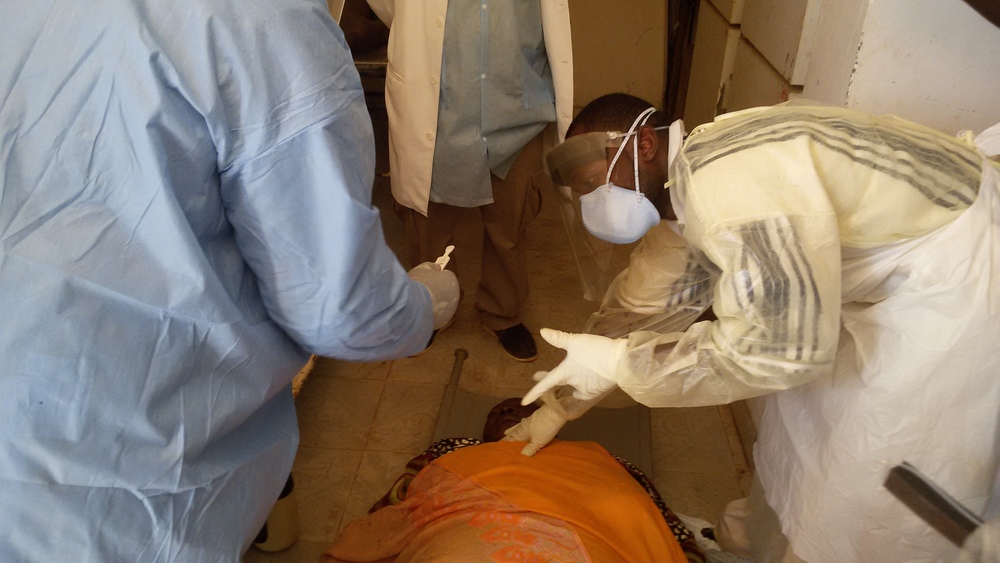
A common cultural practice in Sierra Leone is for a group of family members to wash and prepare the deceased relative before burial. This practice was discontinued by the Government of Sierra Leone (GoSL) during the Ebola outbreak in an effort to curb transmission. Safe and Dignified Burials (SDBs) were introduced by the GoSL as an alternative burial method for family members by qualified and properly attired health care workers. The Ebola outbreak has significantly decreased, so the GoSL has began to allow family members to bury loved ones as long as the individual has not met the Ebola case definition before expiring. A swab of the body is taken before the body is approved for burial and delivered to a regional laboratory for testing. Due to a lack of resources, swab laboratory confirmations can take 2 - 5 days on average before the family is informed of the result, and sometimes longer. The benefit of point-of-care tests like OraQuick, is that family members can be notified of presumptive results almost immediately, which enables the deceased to be released with confidence to family members within the same day.
OraQuick is easy to perform and requires only a drop of blood from a finger prick or swab from the oral cavity of a live patient. A swab from the oral cavity of the deceased may also be used for testing. The tests can be performed onsite, require minimal specialized training to perform, and produce a presumptive result in only 30 minutes. The RST has received U.S. FDA’s Emergency Use Authorization approval. When testing deceased bodies, only OraQuick has been cleared for use.
The goals for the training and implementation of RST testing was as follows:

-
Provide a reliable, simple, and rapid Ebola testing technology option.
-
Improve compliance with dead body swabbing which enhances surveillance.
-
Provide alternate technology for presumptive POC diagnosis of suspect EVD cases, and manage more effectively the number of samples submitted for Ebola RT-PCR analysis.
The training was highly effective, and produced the following results:
-
38 DSOs, swabbers, and surveillance officers were trained on the OraQuick Ebola RST Protocol.
-
Participants were refreshed and re-trained on proper PPE protocol and procedures
-
The training confirmed the existing surveillance system supports the developed RST protocol.
-
Surveillance teams in the Western Area and Kenema will collect OraQuick Ebola RSTs over the next three weeks.
The swab teams will receive certificates of completion at the end of the three week pilot from eHA and the CDC. They directly engage with local communities during the testing process and have received positive feedback and willingness from households and family members to have their loved ones tested for Ebola using the RST device.
